What is Caffeine?
The word “caffeine” originated from the German word “kaffee” and the French word “café”, both directly translating to mean “coffee.” Caffeine’s chemical name is 1,3,7-trimethylxanthine, based on its formula, C8H10N4O2, and molecular structure. Caffeine is a naturally occurring stimulant that can be isolated from over sixty plants, but can also be made synthetically and added to our everyday food products and medications. In its pure form caffeine is a white powder that is very bitter.
products and medications. In its pure form caffeine is a white powder that is very bitter.
Much of the modern population uses caffeine as a stimulant, exciting the brain and nervous system while fighting fatigue. Making caffeine one of the most widely consumed psychoactive agents in the world!
Caffeine is a drug like substance that shares traits with other addictive substances such as amphetamines, cocaine, and heroin. Caffeine uses the same biochemical mechanisms as these other drugs to stimulate brain function. As a result of excess caffeine consumption you may feel like your mind is racing and your thoughts are coming much faster, this feeling can at times be very overwhelming.
To combat the sense of “turbo-speed” decaffeinated products are now more widely consumed. Through extraction processes, that are explained in more detail below, individuals can enjoy the taste of beverages, like coffee, without feeling overstimulated.
History
Caffeine has been a part of our global history for thousands of years. Each country has  its own story and source of caffeine. One of the most eccentric caffeine findings was in Ethiopia. The folk stories passed between generations says that a farmer, who had recentlly moved his goats to a new pasture found them to be restless and ancy. For the next few days he watched them, and noted that they were grazing on small berries. These berries were later dried and called “coffee beans.”
its own story and source of caffeine. One of the most eccentric caffeine findings was in Ethiopia. The folk stories passed between generations says that a farmer, who had recentlly moved his goats to a new pasture found them to be restless and ancy. For the next few days he watched them, and noted that they were grazing on small berries. These berries were later dried and called “coffee beans.”
Isolation
Caffeine was first extracted from cocoa beans into its purest form, a white powder, in the 1820s by a German Scientist named Friedrich Ferdinand Runge. Today caffeine is easily extracted and used to make a variety of products that are consumed on a daily basis.
Extracting caffeine from coffee to produce decaffeinated coffee and a caffeine powder can be done many different ways. A few of these processes are no longer used because of the health risks, environmental impact, cost, and flavor changes that were associated with the solvents. Common solvents that were used include Benzene, chloroform, trichloroethylene, and dichloromethane. There are now three main processes that have superseded the use of the previous solvents.
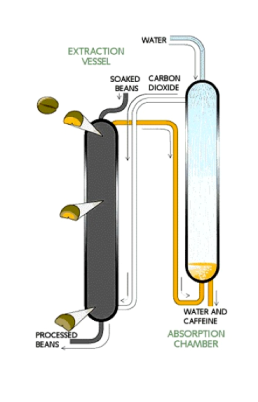 Water extraction: Raw, green coffee beans are soaked in distilled water for an extended period of time. The water, containing many other compounds including caffeine and flavor compounds, is then passed through a charcoal filter. The charcoal removes caffeine from the solution, leaving the flavor compounds. The solution is then placed back with the original coffee beans and allowed to evaporate, leaving decaffeinated beans with their original flavor. Many manufacturers collect the caffeine from the charcoal filter and resell it for use in products such as soda and over the counter medications.
Water extraction: Raw, green coffee beans are soaked in distilled water for an extended period of time. The water, containing many other compounds including caffeine and flavor compounds, is then passed through a charcoal filter. The charcoal removes caffeine from the solution, leaving the flavor compounds. The solution is then placed back with the original coffee beans and allowed to evaporate, leaving decaffeinated beans with their original flavor. Many manufacturers collect the caffeine from the charcoal filter and resell it for use in products such as soda and over the counter medications.
- Supercritical Carbon Dioxide extraction: Carbon dioxide, CO2, is a great solvent that is safer than the organic solvents used in previous extractions. The process begins when CO2 is forced through green coffee beans at a temperature above 31.1 °C and a pressure above 73 atm. Under these conditions, CO2 enters a “supercritical”state. In a supercritical state it has gaslike properties, that allow it to penetrate deep into the beans, while maintaining its liquid-like properties that allow it to dissolve 97–99% of the caffeine in the beans. The CO2, now containing caffeine compounds, is then sprayed with high pressure water to remove the
 caffeine. This water can then be filtered and caffeine can be isolated into its purest white powder form.
caffeine. This water can then be filtered and caffeine can be isolated into its purest white powder form.
- Extraction by organic solvents: Research has found certain organic solvents, such as ethyl acetate, that have a lower health risk and environmental hazard than chlorinated and aromatic organic solvents used years before. These solvents are used to dissolve caffeine, and are then rinsed away leaving the coffee been decaffeinated.

Pingback: Home | Caffeine
Pingback: Coffee - All About Coffee and Caffeine - ENT Wellbeing Sydney
Pingback: Caffeine and How it Affects You
thxs for the examples